What is polyethylene? Indeed, what is the secret of this popular plastic among people of the world, which has 34% of the consumption share of all polymers in the world? Perhaps the German scientist “Hans von Pachmann” himself did not think that this wax-like substance, which he accidentally “synthesized” by heating synthesized, would reach this level of popularity in the world.
list of content
1: Primary synthesis of polyethylene
2: Classification of polyethylene based on density
3: The structure of the ethylene molecule
4: The structure of the polyethylene molecule
5: The color of the polyethylene material
6: Molecular weight and crystallinity of polyethylene
1: Primary synthesis of polyethylene
It is interesting to know that, the first method of industrial synthesis of polyethylene; It happened accidentally by two scientists named Gibson, Reginald and Eric Fawcetts in March 1933. This experiment, which led to the invention of polyethylene, was carried out by heating ethylene and benzaldehyde at a temperature of 170 degrees Celsius and a high pressure of up to 2000 atmospheres using very basic equipment.
The material that was obtained as a result of this reaction was a white wax-like solid, which was named polyethylene some time later when this method was perfected and developed.
This method was developed by “Michael Perrin”. By synthesizing polyethylene under high pressure, he found a fundamental method for the industrial production of low density polyethylene (LDPE).
2: Classification of polyethylene based on density
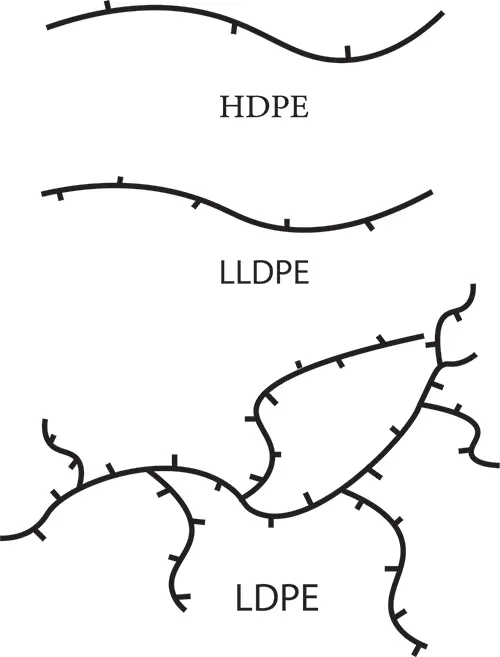
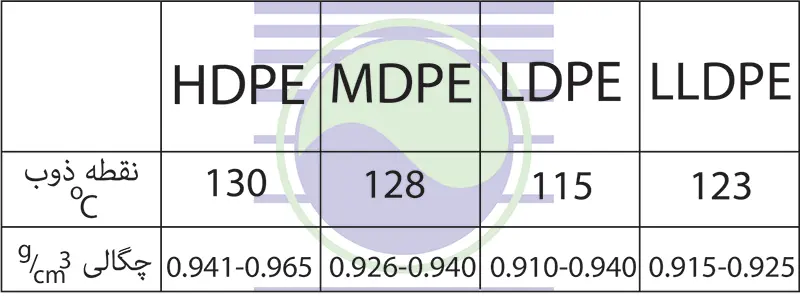
Based on the density, polyethylene is divided into four main categories: low density polyethylene LDPE, medium density polyethylene MDPE, high density polyethylene HDPE and linear low density polyethylene LLDPE.
LDPE low density polyethylene:
Low density polyethylene (LDPE) is a linear polymer with short branches. The density of this polymer is between 0.910-0.940 g/cm3 (grams per cubic centimeter). The usual method of producing this polymer is by copolymerization with short chain alpha-olefins such as butene-hexene-octene.
LDPE low density polyethylene has high degree of branching of short and long chains, high softness and flexibility, low moisture absorption, good chemical resistance, easy welding are the characteristic features of this type of polymer.
This polymer is mostly used in the production of films and nylons for the packaging of various goods due to its high flexibility and high resistance to puncture, impact and stretching. Examples of products produced by this polymer include agricultural nylons, transparent films for greenhouses, soft drink bottles, sauce bottles, children’s toys, container lids, wire and cable insulation, etc.

Polyethylene production pipes size 16 to 20 mm of Petroab Hayat Kala Industrial Group are included in this category.
The low density polyethylene recycling code is four; This means that recycling low density polyethylene is more difficult than high density.

MDPE medium density polyethylene
This polymer, whose density value is between low density and high density polymer, can be produced with the help of chromium and silica catalysts. Brier’s resistance to cracking and impact can be mentioned among its characteristics.
Medium density polyethylene (MDPE) is also used in many industries, including automobile manufacturing, household appliances, as well as for making gas supply pipes and fittings, Caps of packaging containers, etc. The density of this grade of polyethylene is 0.926-0.940 g/cm3 (grams per cubic centimeter).
Petroab Hayat Industrial Group has used this grade of polyethylene for the production of pipes and fittings in many projects of Iran’s National Gas Company.

HDPE high density polyethylene
High density polyethylene HDPE is much easier to recycle than low density polyethylene LDPE and its recycling code is 2. Another name known among the people is alkatan and polythene. This grade of polyethylene has many uses and also has been used in various industries. Milk bottles, gallons of chemicals, high pressure pipes of oil and gas lines, etc. are among the products produced in this polyethylene grade. The highest density value is usually related to this polymer, which is from 0.941 to 0.965 (grams per cubic centimeter) g/cm3.

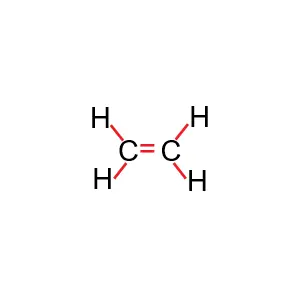
The structure of the ethylene molecule
Ethene or ethylene is the main molecule of polyethylene polymer, which we know by the formula C2H4.
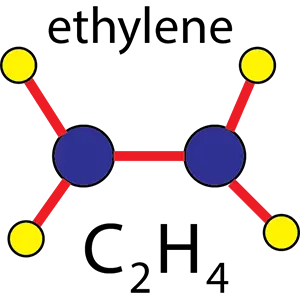
In the image below, you can see the ethylene molecule in three dimensions.
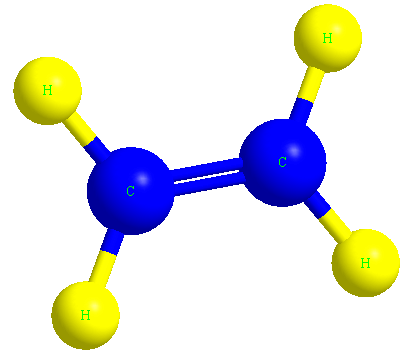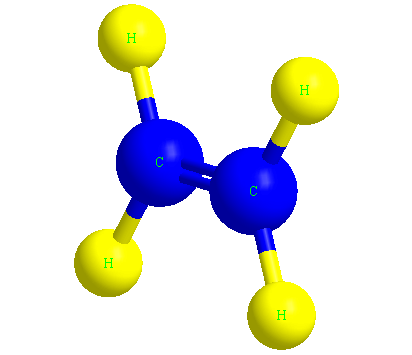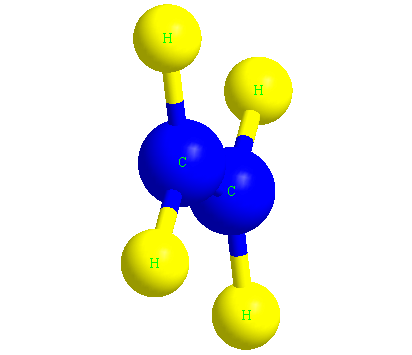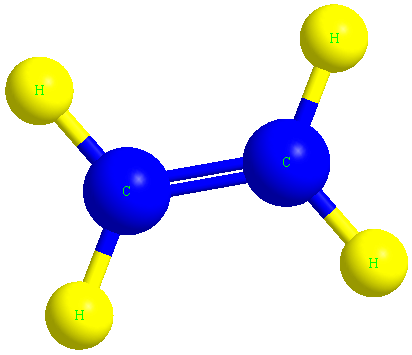
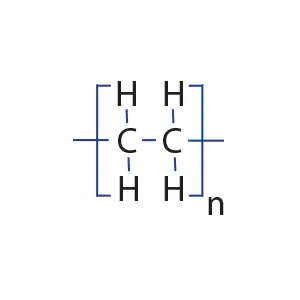
The structure of the polyethylene molecule
When ethylene molecules are connected to each other in a chain, they form a polymer called polyethylene. In fact, polyethylene is considered a macromolecule that consists of a number of smaller units or monomers called ethylene. The word polymer has a Greek origin, and its Persian equivalent is Baspar. (poly) means many and (mer) means part or unit, this word was first used in 1835 by a chemist named Renault.
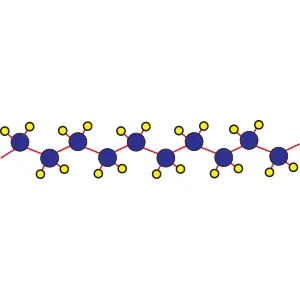
In the picture below, the macromolecule of polyethylene is completely visible in three dimensions.
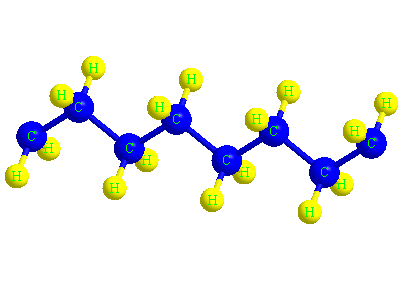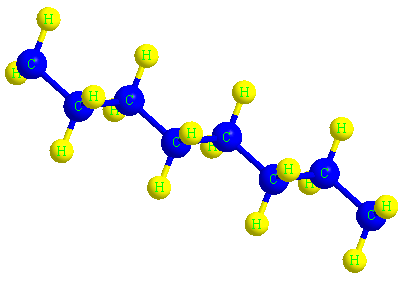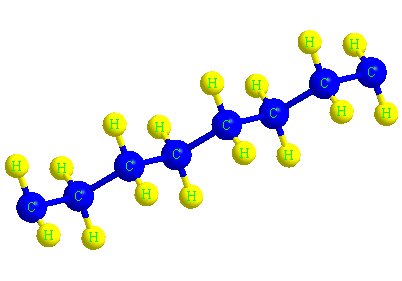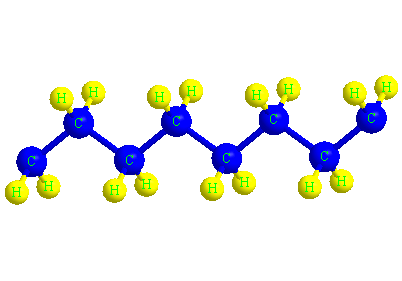
The color of the polyethylene material
Polyethylene itself is colorless and transparent and the reason for seeing black color in polyethylene pipes is the presence of 3 to 5% carbon black, which will make it more resistant to UV rays.

This white substance is produced as a result of the polymerization of ethylene gas under high pressure and temperature. It should be noted that a catalyst is needed for the production of polyethylene, and the most widely used of these catalysts is a combination of aluminum alkyls and titanium halides called Zigler Natta.
Molecular weight and crystallinity of polyethylene
Molecular weight and crystallinity are the most important factors that determine the properties of polyethylene types, because the determinants of these properties depend on the molecular structure.
Crystallinity has an opposite side with molecular weight and branches of the polymer chain, so that the lower the molecular weight and the fewer branches polymer chain has, the higher crystallinity.
You can see the degree of polyethylene branching types in the figure below: